How to Find Mass Percent of Acetic Acid in Vinegar
Finally the mass percent of acetic acid in the vinegar can be determined from the mass of the acetic acid in the sample and mass of the vinegar solution that was titrated. Since mass moles X molar mass.
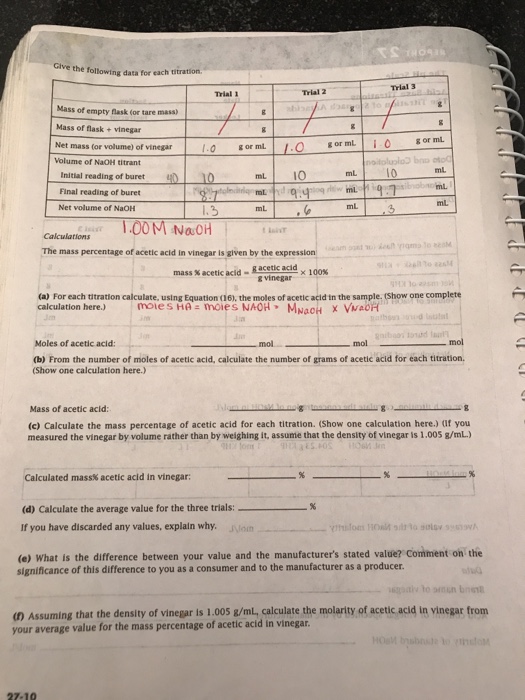
Solved Give The Following Data For Each Titration Chegg Com
How much acetic acid is in white vinegar.

. The mass of acetic acid in vinegar from trial 3 was higher than the other two trials included in the experiment with it being 6. Mass of HC 2 H 3 O 2 1 L. Mass acetic acid in vinegar 13097 g volume of acetic acid mass acetic acid density acetic acid volume acetic acid 13097 1049 1249 mL Calculate concentration of acetic acid in vinegar as vv acetic acid in vinegar vv volume of acetic acid volume of vinegar 100.
Vinegar is a solution of acetic acid CH 3 COOH or HC 2 H 3 O 2 in water. How do you calculate acetic acid in vinegar. How many grams of acetic acid are in 100mL of vinegar.
When used for pickling the acetic acid content can be as high as 12. The density of vinegar is pu1106 g mL-1. If we assume the density of vinegar is very close to 100 gmL then the mass of 100 L of vinegar is 1000 g.
Titration is a common method used by chemists to find the concentration of a substance in a solution. White distilled vinegars are generally 4-7 acetic acid. Cider and wine vinegars are 5-6 acetic acid.
In this experiment you will determine the mass percent of acetic acid in vinegar by titration. 02844 m o l 1 L 6005 g m o l 1 1 L 1000 m L 0017 078 22 g m L 1. How many grams of acetic acid are in a liter of vinegar.
A vinegar sample is found to have a density of 1006gmL and to contain 87 acetic acid by mass. Calculate the mass of acetic acid in the vinegar in the previous exercise question1 above. The label on the bottle of vinegar states the percent acetic acid in vinegar is 500.
Mass percent of acetic acid. Often the equivalence allude is figured out visually with an indicator. In the mass percentage of acetic acid in vinegar will be determ vinegar with a sodium hydroxide solution of known concentration.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. On 19 The experimental value of the mass percent acetic acid in vinegar is 412. What is the average molarity of acetic acid in vinegar.
Acetic acid however is NOT synonymous with. 5 mass. The concentration of the sodium hydroxide solution used to titrate the acetic acid was determined to be 1 M.
Using average molarity given pu02844M calculate the mass percent acetic acid in vinegar for comparison to the stockroom claimNot sure how to approach the problem but this is what Ive donefracpu02844 molpu1L times. 5087 5089 5083 3 5086. The density of vinegar is 1106 gmL1.
Calculate volume of acetic acid using its mass and known density density gmL mass g volume mL. The purpose of this experiment is to determine the acetic acid content of. Calculating the Concentration of Acetic Acid as a Percentage By Volume vv Extract the relevant data from the experiment volume of acetic acid.
M a 008478 M of acetic acid. The titration method proved to be easy to perform and fairly precise in measuring concentrations of acids and bases. 5087-5086 2 5089-5086 2 5083-5086 2 3-1 0003.
Rinse the buret thoroughly with distilled water then clamp it to a ring stand. Mass of vinegar solution titrated. The grams of acetic acid in the vinegar sample can be calculated by multiplying the moles of acetic acid by the molecular weight of acetic acid MW.
The indicator i beg your pardon is a substance that alters color near the equivalence point is added to the analyte solution. 008478 x 60 5087 Repeat for other trials. Calculate mass of acetic acid mass moles molar mass.
Using average molarity given 02844 M calculate the mass percent acetic acid in vinegar for comparison to the stockroom claim. - mass of acetic acid in 500 mL vinegar 067 X 60 402 g. The percent by mass of acetic acid in the vinegar sample can be found from Equation 1.
To determine the average mass of acetic acid the mass of acetic acid in each trial were added together and then divided by the number of trials. CHCOOH aq NaOHaq - CHCOONa aq HO 1 PROCEDURE 1. Mass of acetic acid in sample.
The percent by mass of acetic acid can be expressed as. The label on the bottle of vinegar states the percent acetic acid in vinegar is 500. Not sure how to approach the problem but this is what Ive done.
You are watching. I 2 n 1. The amount of acetic acid is usually 5 by mass in the vinegar solution.
The stockroom claims the percent acetic acid in vinegar to be 20. The stockroom claims the percent acetic acid in vinegar to be 20. Similarly it is asked how many grams of acetic acid are in 100mL of vinegar.
Actual Experimental Actual x 100 5086 5 5 x 100 17. Conclusion The percentage of acetic acid in vinegar was determined to be 656 in the first type of white vinegar and 8263 in white wine vinegar. More generally vinegar can be defined as a solution composed of acetic acid HC2H3O2 water and perhaps other substances.
Using average molarity given 02844 M calculate the mass percent acetic acid in vinegar for comparison to the stockroom claim. The acetic acid content of vinegar can vary widely but for table vinegar it typically ranges from 4 to 8 vv. The molarity and mass percent of the vinegar are 08393 molL and 5010.
How to find mass percent of acetic acid in vinegar.

Csus Ch6a Mass Percentage Of Acetic Acid In Vinegar Instructor
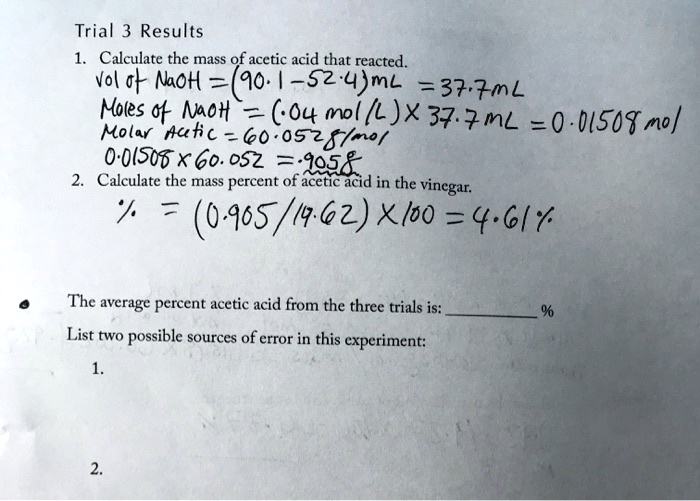
Solved Trial 3 Results Calculate The Mass Of Acetic Acid That Reacted Vol Noh 90 5z 4 Ml 37 7nl Moles Moh 7 Co4nl L Jx37 Nl 0 0 Sot No Molar Aukc 60 0525 0

Solved Analysis Of Vinegar Trial 2 Trial 1 Volume Of Vinegar Chegg Com
No comments for "How to Find Mass Percent of Acetic Acid in Vinegar"
Post a Comment